Originally published in LabTalk (Volume 2, Issue 3, 2007), the quarterly newsletter of Aculabs.
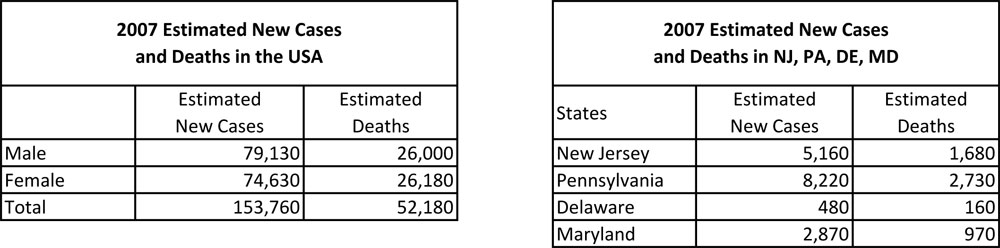
Cancer is the second-most frequent cause of death in the United States, and Colorectal Cancer (CRC) is the third most common cancer in the US and the world. In a recent statistical study from the American Cancer Society, CRC was the second leading cause of cancer death, and it is estimated that 153,760 new cases (51.46% male, 48.54% female) will be diagnosed, with 52,180 deaths by the end of the year 2007. Most of the CRC cases are located in the rectum followed by sigmoid, cecum, transverse colon and flexures, ascending colon, and descending colon.
Early colon cancers rarely display any symptoms, which make the screening very important. With early detection, localized stage, and appropriate treatment the 5-year survival rate is 90%. Unfortunately, only 37% of the incident cases are diagnosed while it is localized.
The American Cancer Society (ACS), US Preventive Service Task Force (USPSTF), and the National Academy of Clinical Biochemistry (NACB) recommend that all subjects 50 years old or older (high risk patients and patients with inflammatory bowel diseases should be tested earlier) undergo screening for CRC using one of the following methods:
- Fecal Occult Blood Test (FOBT) every year
- Sigmoidoscopy or double contrast barium enema every 5 years
- Colonoscopy every 10 years
The most widely used test for screening in the asymptomatic population is FOBT which offers low cost, non-invasive testing that can be done at home and sent to the lab. Although FOBT does not decrease the incidence of CRC, it reduces the mortality very significantly. In several studies, the mortality was much as 33% lower in the population that had at least one FOBT screening than in the control group. In an effort to provide our clients with the latest and the best available assay, we evaluated a new methodology to measure FOBT. It is an automated immunoassay, detects human hemoglobin which makes it less subject to interferences (dietary or medicinal), and only one specimen required.
The sensitivity and specificity of the assay was excellent, and offers several advantages over the Guaiac method (the old method) including: no interference with any substances that are known to affect the Guaiac method (the old method) like vitamin C, iron and peroxidase. Only one specimen is needed and the major advantage is decreasing anxiety for patients and the number of unnecessary follow-up procedures by decreasing false positives. This assay is available to our clients 5 days/week, and we will be using this method as the first choice and default when the assay type is not specified. You must specify “Guaiac Method” if desired.
– Dr. Rita Khoury
[hr]
Have a topic you’d want our Lab Director to cover in future posts? Send suggestions to info@aculabs.com!

Leave A Comment
You must be logged in to post a comment.